Abstract
At present, there is no consensus on whether and how to treat children with molecular relapse of Philadelphia-Positive (Ph+) Acute Lymphoblastic Leukemia (ALL). A " watch and wait " strategy is the current approach until evidence of hematological recurrence for which there is neither a specific and validate treatment.
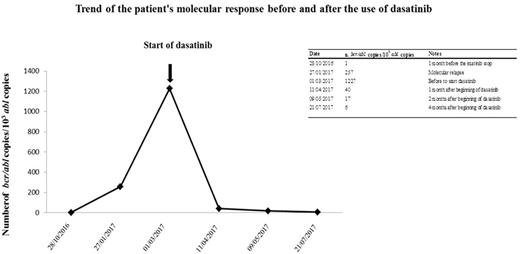
We herewith describe the case of a 17.5 years old boy with Ph+ ALL, treated at diagnosis according to the EsPhALL protocol, at low risk, with imatinib at the dosage of 300 mg/mq daily, given continuously from day 15 of Induction until treatment discontinuation. He reach clinical remission at the end of Induction and BCR-ABL1 /10.000 ABL transcript copies progressively reduced from 28,975 at diagnosis to 1 at the end of Induction and negativity of IG/TR molecular minimal residual disease (MDR). After 2 years of treatment and 2.5 years of imatinib, at the end of all therapy he showed 1 copy and negative MRD. Two months after imatinib discontinuation a molecular relapse was observed (257 copies of BCR-ABL1/10.000 ABL). One month later, bone marrow evaluation showed a marked and rapid increase of the fusion gene copies (1227 BCR-ABL1/10.000 ABL copies); conventional cytogenetic was positive (n. 3 of 20 metaphases analyzed) and IG/TR molecular MDR was 1x10-4, consistent with the increase of transcripts copies.
The second-generation tyrosin-kinase inhibitor dasatinib is effective and safe in children, adult and elderly patients affected by Ph+ ALL and it is considered a suitable option in adult ALL resistant or relapsed after imatinib. However, the experience in treating molecular relapse with dasatinib in children is anecdotal. In order to prevent an hematological relapse and to obtain a new molecular remission, dasatinib treatment was purposed.
One month after molecular relapse, a written informed consent was obtained and dasatinib was started, with a dose of 60 mg/mq daily, associated to prednisone (20 mg/mq daily) for the first 21 days, after which dasatinib was given alone at the same dosage. After 1 month of therapy the patient obtained a cytogenetic remission and a rapid decrease of BCR-ABL1 copies, with 40 BCR-ABL1/10.000 ABL copies, further reduced to 17 copies one month later and 6 copies at the last control, after two months, in July 2017. Currently, the boy is continuing dasatinib, without any side effects and enjoying a good quality of life.
Although it is a single case report with a short follow up, we think it is important to share a positive experience on the use of dasatinib after molecular relapse in a children with Ph+ ALL, which resulted rapidly active and well tolerated.
It remains to be defined how long to continue the treatment after obtaining the molecular remission and whether this therapy is sufficient to prevent the hematological recurrence.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal